Abstract
This paper reviews current concepts and results in the management of congenital tracheal stenosis (CTS). Diagnostic options are considered and the requirements for successful management defined. Chief amongst these is a multidisciplinary approach with individualised patient management.
Severe long-segment CTS represents the biggest challenge to clinicians and the worst problems for affected families. Near-death episodes are frequent in affected infants and some cannot be ventilated and require Extra-Corporeal Membrane Oxygenation (ECMO). Associated cardiovascular anomalies are frequent. Patients require immediate resuscitation and transfer to a specialist unit.
After careful assessment, accurate diagnosis, and discussion, primary resection and end-to-end repair with a slide technique should always be the first option, with concomitant repair of associated cardiac anomalies. If this is impossible because of the severity of the lesion, some form of patch tracheoplasty will be indicated. Cardiopulmonary bypass is often required. Patches include pericardium, autograft trachea, carotid artery, cartilage, and allograft trachea.
Mortality ranges from 0% to 30% in the literature, which largely comprises single-centre long-term experience. Recurrence is common and can be managed by stenting and tracheal homograft implantation. Long-term quality of life of survivors is little reported but seems good. Physiological data are lacking.
To improve results, we suggest a treatment algorithm to rationalise care.
Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from Research and Development funding received from the NHS Executive.
E-mail address: [email protected]
Popular choices
- Casinos Not On Gamstop
- Casinos Not On Gamstop
- UK Betting Sites
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- Non Gamstop Casinos UK
- Slots Not On Gamstop
- Sites Not On Gamstop
- UK Casino Not On Gamstop
- Non Gamstop Casinos
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- Casinos Not On Gamstop
- Top UK Slot Sites
- Casinos Not On Gamstop
- Non Gamstop Casino Sites UK
- Sites Not On Gamstop
- Casino Sites UK Not On Gamstop
- Casinos Not On Gamstop
- Casino Sites Not On Gamstop
- UK Casinos Not On Gamstop
doi:10.1016/S0531-5131(03)01039-2
Click here for the PDF version
Contents
1. Introduction
Congenital tracheal stenosis (CTS) describes a spectrum of disorders. It is rare, but often life threatening and may be difficult and expensive to treat. It can be very distressing for the patient, their families, and for their carers. Symptoms and signs usually occur in the first months of life. Depending on its severity, it may present with simple stridor or with near-death episodes requiring resuscitation at home, or worse, inability to ventilate at all and thus require immediate Extra-Corporeal Membrane Oxygenation (ECMO) support [1].
There is no standard definition of LSTS. The reports in the literature describe, by means of diagrams, several variants LSTS. In our own practice (largely, but not exclusively congenital) over the last few years, we have begun to understand the significance of this variation. There are at least four elements that imply severity.
- The narrowness of the trachea. We have seen several small infants whose trachea is so severely narrow that ventilation is impossible and ECMO support has been required. There is a big clinical difference between a trachea of 1 mm internal diameter and one of 2.5 mm internal diameter.
- The extent of tracheal involvement. This is probably the classic description of severity, often stated in terms of 'thirds' of the trachea. LSTS usually implies greater than two-thirds of the trachea is involved.
- The involvement of the bronchi. This feature is often ignored, yet may militate against the use of particular types of repair. In very small babies, especially those with associated cardiac lesions, the bronchi are often involved. Associated bronchomalacia has emerged as a significant risk factor in those with extensive disease.
- The presence or absence of complete tracheal rings. Complete tracheal rings are common in congenital LSTS. Although they may grow and there are many children alive with complete tracheal rings, some seem to prevent adequate growth and precipitate severe symptoms.
We think that all patients with tracheal stenosis should have their tracheas described according to these terms. Morphologic description should also define associated morphologic anomalies of the airway (e.g., pig or eparterial bronchus, hypoplasia or aplasia of the lung) and other associated anomalies particularly of the heart. Each team seeing these patients will have a specific referral population related to its particular skills. For example, at least 40% of our patients have associated cardiac anomalies, reflecting our role as a quaternary cardiac referral unit.
Interpreting the available literature is difficult because of inconsistent definitions and the lack of agreed standards of severity. Many authors, however, have resorted to pictorial descriptions of the treated lesions [2]. Until adequate definitions are agreed, these methods are very helpful.
Relatively few authors have contributed relatively few patient histories to the literature, mostly in the last 20 years. This reflects the rarity of the disorders, as well as their complexity and difficulty in management. These considerations argue strongly in favour of specialist units and multidisciplinary dedicated teams. As we will make clear, intense commitment is required to care for these patients.
2. Presenting symptoms and management
A substantial minority of the patients we are referred1 have only severe inspiratory and expiratory stridor as their primary symptoms. Such patients can often be investigated as an outpatient. The majority, however, have presented to their local hospital in extremis after 'collapse' at home. Active resuscitation by parents has frequently been required, and most have been intubated with difficulty and prove hard to ventilate. Ventilation with helium as an inert oxygen carrier can be helpful to reduce drag on gas flow and reduce air trapping. Some patients have proved impossible to ventilate and have needed ECMO. A common statement from referring physicians is that the patients 'keep trying to die'.
As soon as the patient's ventilatory state is made 'stable,' the child should be transferred to a specialist unit. Transfer can be extremely hazardous and, in our view, should be made by a trained paediatric retrieval team.
3. Investigation
Once the patient is in the appropriate environment, careful assessment and investigation are mandatory. We perform echocardiography, fibre optic bronchoscopy, and bronchography in all patients. Echocardiography is mandatory because of the frequency of associated cardiovascular malformations. Computed tomography (CT) or magnetic resonance imaging of the chest is often helpful to assess vascular anatomy. This is important, as more than half of our patients with tracheal stenosis have associated vascular anomalies, most often a pulmonary artery sling [3, 4, 5, 6]. Virtual bronchoscopy and three-dimensional reformatting of CT images [7, 8] may be useful to show the extent and severity of stenosis, but have significant shortcomings. In particular, they do not allow dynamic evaluation of associated bronchomalacia, and they may not have sufficient spatial resolution to show subtle but important anatomical findings such as complete tracheal rings. In very small children, transmitted cardiac pulsation causes an inevitable degradation in image quality. Although electron beam CT scanners may avoid this pitfall [9, 10], these are not widely available in children's hospitals. It is for these reasons that we continue to perform bronchography, a simple and cheap procedure which is easily combined with bronchoscopy and which has excellent spatial and temporal resolution.
Microlaryngobronchoscopy may be needed to assess the larynx and the segment of the trachea above the stenosis if there is any question of obstruction at more than one level. Angiography is now rarely indicated for the assessment of vascular anomalies associated with tracheal stenosis.
4. Management at the specialist centre
The primary management must be to stabilize the airway. It is almost never necessary to perform a tracheostomy, but ECMO support has been necessary in four of our patients over the last 6 years.
After completion of investigations, detailed discussions must take place between the management team and the family. All treatment options must be presented, and stress must be laid upon the uncertainty of long-term outcome and the limited data available on which to give advice. The management of CTS imposes enormous strains on family relationships, and these must be taken into account during preoperative counselling. These anticipated family consequences prompt some families to opt for no treatment. Support from a liaison nurse and or social workers is often needed. Long hospital stays are not uncommon and may be far from the patient's family home. This adds significantly to the stress.
The management plan is usually based on the severity and extent of the stenosis and the nature of associated malformations. For the purposes of this paper, it should be assumed that associated lesions would always be repaired concomitantly with the trachea.
5. Management options and results
Management is categorised according to the severity and extent of the stenosis. Whenever possible, associated cardiac lesions should be simultaneously repaired.
5.1. For very short-segment stenoses
Classically primary resection and end-to-end anastomosis is the treatment of choice [11]. In the last 6 years, we have done four such repairs with good outcomes, except in one boy who had persistent localised malacia requiring tracheostomy support.
Recently, balloon dilatation has become an accepted modality [12, 13, 14, 15] and the results are encouraging. This can be combined with posterior lasering to release complete tracheal rings [16]. Not enough patients have been done to recommend this method, but it is undoubtedly worth exploring. Various stents have been employed [17, 18, 19], but we can see few indications for their use in children, where definitive repair is of greater value, permitting rather than constraining growth as is inherent in the use of stents.
5.2. For medium-length (<2/3 trachea) stenoses
For these lesions, we agree with Grillo [2] that slide tracheoplasty should be the initial treatment of choice. Slide tracheoplasty was first described by Goldstraw's group at the Royal Brompton Hospital, London [20] and later popularised by Grillo from the Massachusetts General Hospital [2, 21]. In the slide tracheoplasty, the stenotic segment is transected at its midpoint, the upper and lower segments are incised vertically (anteriorly in one segment, posteriorly in the other), the corners of the segments are trimmed to spatulate them, and the two ends are slid together and sutured. As Grillo has pointed out and as has subsequently been confirmed by Macchiarini et al. [22] in animals, the circumference of the trachea is doubled and the cross-sectional area quadrupled. Only native tissue is used, and normal, ciliated tracheal epithelium is immediately present. Subsequent tracheal growth has been seen to be satisfactory.
In children, mobilisation of the trachea is usually remarkably easy, and it is usually equally easy to perform the slide tracheoplasty. Because of the excellent mobilisation, it is even possible to remove the entire trachea in infants [23], although later reconstruction may be required.
Despite these theoretical advantages and the obvious logic which they describe, there are surprisingly few data in the literature to support the contention that slide tracheoplasty should be the definitive treatment for LSTS. Grillo's recent report [2] includes a table listing the relevant publications and the number of patients reported. We have modified that table (Table 1) to include more recent publications and our own centres' (GOS team) 5-year experience. These reports cover a wide range of patients from infants to age 43 years. The limited numbers of patients infer limited application of the technique, even in centres operating on a lot of patients with tracheal stenosis. Some case selection must be taking place.
| Reference | Year | Number | Deaths |
|---|---|---|---|
| Tsang et al. [20] | 1989 | 2 | 1 |
| Dayan et al. [42] | 1997 | 2 | 1 |
| Houel et al. [43] | 1998 | 1 | 0 |
| Muraji et al. [44] | 1998 | 1 | 0 |
| Lang et al. [45] | 1999 | 2 | 0 |
| Lipschutz et al. [46] | 2000 | 1 | 0 |
| Matute et al. [28] | 2001 | 4 | 0 |
| Garabedian et al. [46] | 2001 | 1 | 0 |
| Grillo et al. [2] | 2002 | 8 | 0 |
| Acosta et al. [47] | 2000 | 3 | 1 |
| Kutlu et al. [24] | 2002 | 1 | 0 |
| GOS team | 2002 | 6 | 1 |
| Total | 32 | 4 (12.5%) |
It is obvious that slide tracheoplasty is both possible and effective. Other than Grillo's report, little is known about long-term results, although Goldstraw [24] has reported an excellent long-term outcome in his index case.
However, there are occasions when it can be difficult or impossible to perform a slide tracheoplasty. In particular, when there is a pig or eparterial bronchus with a significant length of intermediate airway between the false and true carina, the operation can be impossible, and other procedures should be employed.
5.3. For long and very long stenoses
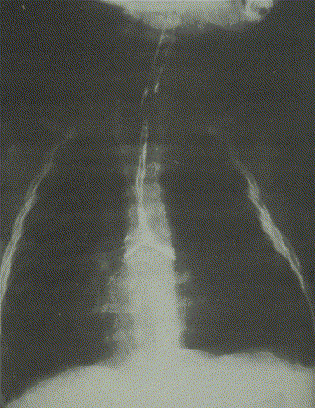
It is for this group that the greatest number of options exist, and for this group that the greatest controversy remains. We believe that resection±slide tracheoplasty should always be the first option. However, not all patients can be treated this way. For patients with very long, very narrow stenoses (see Fig. 1), and especially for those involving the bronchi, some form of patch tracheoplasty is usually indicated.
The basic principles of patch tracheoplasty are simple. CPB is usually necessary. The trachea is opened longitudinally anteriorly from above to below the stenosis. This incision may extend from carina to the bronchial branches. The anterior defect is then enlarged with a patch. Several materials have been employed over the years, but the most common and successful have been autologous pericardium [25, 26, 27], rib cartilage [28], tracheal autograft [29], tracheal allograft [30, 31, 32], and most recently, carotid artery [33, 34]. Heterograft tissue and prosthetic material do not work. Brown's group [25] recommend the use of suspension sutures on the outside of the pericardial patch to create tent-like suspension of the patch. To differentiate this technique from other pericardial patch repairs, we have called it 'suspension' patch tracheoplasty. The available results of these procedures are shown in Tables 1–4. Once again, it is important to stress that there are relatively few data, mostly from single centre series over long eras. Interpretation of such data is bound to be difficult.
| Author | Year | Patients | Mortality |
|---|---|---|---|
| Andrews et al. [48] | 1994 | 13 | 6 (47%) |
| Bando et al. [25] | 1996 | 12 | 2 (17%) |
| Backer et al. [27] | 2001 | 28 | 7 (25%) |
| GOS Team | 2002 | 15* | 4 (27%)* |
| Total | 68 | 19 (28%) |
| Author | Year | Patients | Mortality |
|---|---|---|---|
| Lobe et al. [49] | 1987 | 4 | 1(25%) |
| Tsugawa et al. [50] | 1988 | 5 | 1(20%) |
| Kamata et al. [51] | 1997 | 11 | 5(45%) |
| Oshima et al. [53] | 1998 | 4 | 0 |
| Jaquiss et al. [52] | 2001 | 28 | 7 (25%) |
| GOS Team | 2002 | 0 | 0 |
| Total | 54 | 14 (26%) |
| Author | Year | Patients | Mortality |
|---|---|---|---|
| Backer et al. [29] | 2000 | 6 | 0 |
| GOS Team | 2002 | 3 | 1* |
| Total | 9 | 1 (11%) |
The tracheal autograft is usually used in conjunction with a pericardial patch as described by Backer [29]. It utilises relatively stiff tracheal autograft, obtained by resection of the central part of the narrow tracheal segment, to brace open the involved carina. Pericardium is used for the upper tracheal patch.
Tracheal allograft implantation is essentially a variant of patch tracheoplasty, utilising homograft tracheal tissue. First utilised as a limited patch in adults by Herberhold [30], an otolaryngologist from Bonn, it was adapted, with his help, by our team at GOS for use in longer segment stenoses in children when combined with the use of cardiopulmonary bypass. Trachea is harvested at the end of conventional organ donation procedures. After gross cleansing, involving the removal of trachealis and all other loose tissue, the trachea, bifurcation and bronchi with first divisions on each side are placed in formaldehyde for 14 days. The graft is then transferred to methiolate (thimerosal) for 42 days to remove all antigenic protein, and finally, the graft is stored in acetone to dissolve all fat. The trachea can then be stored for up to 1 year. Standard virology and bacterial tests are done to prevent transmission of diseases. The graft retains rigidity similar to that of fresh trachea. Thus, currently applied tracheal homograft replacement (THR) is essentially chemically stored or 'pickled'.
The operation is almost always reserved for those patients with failed previous tracheal repairs of almost any type; indeed, 95% of homograft replacements have been done in patients who have had previous repairs. The anterior part of the recipient trachea is removed, extending as far distally as necessary (including the bronchi if required). The new graft is washed, trimmed to size, and sutured in place over silastic Dumon stents (Novatech, France). A large lumen can be achieved as the graft can be oversized. Increased blood delivery can be achieved by using strap muscle or rectus abdominis flaps to cover the graft [35].
The Dumon stent can be removed endoscopically at about 12 weeks postoperatively. Epithelialisation with functioning respiratory epithelium has been demonstrated, and long-term function is excellent. Quality of life after recovery from the initial procedure is excellent. There has been no evidence of rejection, no immunosuppressive therapy is used, and no homograft calcification has been seen.
Long-term survival is remarkably good [32], with a 12-year actuarial survival of 70%. Given that almost all of the patients had undergone major tracheal reconstructions in the past, this represents a considerable success. Tracheal homograft replacement has a firmly established place as a fallback procedure after failed initial procedures. The operation can be repeated if necessary.
6. Postoperative complications and their management
These are complex and sometimes difficult operations. Postoperative problems are to be expected and fall into some clear categories.
(1) Those related to repair of associated lesions and CPB. Since the majority of associated lesions are cardiac, these relate to the well-described consequences of CPB [36] or the cardiac lesion. Discussion of these is beyond the scope of this paper.
(2) Granulation tissue formation. Granulations occur as part of the healing process of tracheal epithelium. They may result in important and even life-threatening obstruction to a relatively narrow airway. Clearly, their prevention and management are important issues in paediatric tracheal surgery. Attention to technical detail during repair is probably helpful in reducing the incidence, although there are no firm data to support this contention. We would suggest that wherever possible, sutures should be placed deep to the tracheal mucosa in an attempt to diminish the stimuli to granulation formation. Since granulation tissue will develop at the interface between native and patch surfaces, prevention of granulations has been a target of many workers. We have been very interested in the research from Backer's group in Chicago [37], which has reported animal experiments in which vascular endothelial growth factor was found to reduce the formation of granulations and accelerate the rate of healing.
· The management of granulations is usually described as bronchoscopic avulsion with grasping forceps or lasering. We have observed that both these may prolong the problem. In our own practice, we have found that regular balloon dilation (beginning weekly and thereafter gradually extending intervals) has dramatically reduced the incidence of granulation related airway obstruction.
(3) Malacia. As we have become more aggressive about tackling even the most severe tracheal stenoses, we have become aware that underlying distal malacia can be unmasked. Its management can be very challenging. Three strategies can be employed, as we have described elsewhere [38] in the management of conventional tracheo-bronchomalacia. Prolonged ventilatory support with CPAP may work, but a few patients have required tracheostomy and 'home' BIPAP support for up to 2 years. We remain very nervous about the use of long-term tracheostomy in tracheal reconstruction because of the risk of precipitating either granulation tissue formation or recurrent stenosis. Thus, recently, we have chosen to use stents in several such patients. We use largely Palmaz stents, regularly dilating them and planning, in the long term, to over-dilate them to fracture. We do not any longer use such stents in the presence of extrinsic vascular compression. They rarely work and may erode. Malacia may also develop in the patched segment of trachea. Autologous pericardium is soft and floppy. Backer's group report that the patch will become firm enough to support spontaneous breathing within a few days. This has not been a uniform experience for us, and more than half our pericardial patch patients have required intervention by stenting to deal with severe acquired malacia. Backer's team also claim that the autograft remains relatively rigid. Again, we have contrary experience, and a number of our autograft patients have required stenting. Because of the risk of malacia and to encourage trachealisation of patch tissue, Carpentier's group in Paris has researched [33] the use of autologous aortic tissue as a patch. They have demonstrated metaplasia of aortic to tracheal tissue in animals. The Leiden group in Holland has recently reported (in a case report [34]) the use of carotid artery as a patch. We believe this approach holds much promise in the infant.
· The management of malacia after repair remains unclear. However, we have used the same techniques we describe below for recurrent stenosis, with the exception of balloon dilatation.
(4) Infection. While very rare, infection can be catastrophic. During the initial surgery, the unsterile tracheal contents are exposed to the mediastinum, often during a period of immunologic compromise, CPB. We have seen two patients with severe mediastinitis, one of which later proved fatal.
· Mediastinitis must be taken very seriously in this group of patients. Aggressive debridement and antiseptic irrigation should be tried first, but we believe that early muscle flap insertion is the best way to both deal with the infection and deliver new blood supply to the tracheal patch [39].
7. Recurrence
Unfortunately, recurrent stenosis does occur. Abstracting the true incidence of these events from the literature is hard [40]. Most of the series are published by the primary operating team, and it is frequently the case that follow-up is by other (often ENT) teams in the long term. The more severe and extensive the primary lesion, the more likely some kind of recurrence is to occur. Prevention is better than cure, and our management is devoted to both the early detection and prevention of progress of stenosis. Regular bronchoscopy and bronchography is performed. Our strategies for management of recurrence are now clear.
(1) Recurrent stenosis. At the first sign of significant recurrence, we perform bronchoscopy and bronchography, together with balloon dilatation of the stenotic area. Balloon dilatation can then be performed electively at relatively frequent intervals until the stenotic process stabilises.
In practice, we have usually used stents at the first diagnosis of recurrent stenosis, based on our observation that there is usually an important element of collapse, which would probably not respond to ballooning. The most commonly used stent for recurrent stenosis is the Palmaz. This balloon-expandable metal stent is relatively easy to insert, but difficult and sometimes dangerous to remove. The Palmaz stent has the advantage of dilatability with growth, but the disadvantages of potential incorporation into the tracheal wall and erosion into the surrounding blood vessels. This is particularly likely to occur if a pericardial patch has been used. Three of our patients have suffered stent erosion into the innominate artery or aorta. One was fatal, and another required emergency tracheal homograft implantation [39]. It is this experience which leads us to want to explore the use of carotid artery as a patch.
(2) Extensive recurrence, or failed stenting. Under these circumstances, we would perform a tracheal homograft repair as indicated above. It represents an excellent fall-back position, can be repeated, and there is readily available pool of graft tissue.
8. Follow-up
Surprisingly few long-term data are available. Most papers have been published in the last decade, and only a few of them report long-term outcomes. Grillo [2] and Goldstraw [24] emphasise the satisfactory long-term results of slide tracheoplasty, albeit from a group with relatively favourable anatomy. Backer et al. [41] also produce good long-term results from their more complex group of patients. It appears that if a child gets over the initial procedure (this can take months) and provided that the trachea grows, late airway function can be very good. There are no long-term detailed physiological studies. They are badly needed.
Since there are so few data, it is vital that families are aware that they sign a contract with uncertainty when they give consent for treatment. Detailed, careful follow-up by a committed and informed team is vital to provide advice to the child and family and adequately to inform our decision making in the future. In our own practice, all relatively local patients are followed up by a respiratory physician (CW), and all patients living distantly are regularly contacted by the nurse coordinator of the team (CN).
9. The future
There are a number of important areas of research currently underway which will impact on this field. Firstly, initial resuscitation will be helped by advances in helium-based ventilation. Secondly, new patch materials will be developed in the near future. As well as arterial patches, progress in tissue engineering is now very rapid, and groups in Germany and the USA are competing to produce the first engineered 'trachea'. In Japan, most effort has gone into establishing the role of homograft tracheal transplantation, not by chemical storage, but by cryopreservation. It has already been shown in animals that cryopreserved tracheas can be successfully implanted without immunosuppression, and this work should be watched with interest.
Stent technology is also likely to progress fast. Experience with stents in the vascular bed is growing, and there may be a role for drug-eluting stents within the trachea. We await a good absorbable stent for use in children.
A great deal of research is needed into the physiological and social quality of life of these children and their families. Current data are simply inadequate.
10. Conclusions
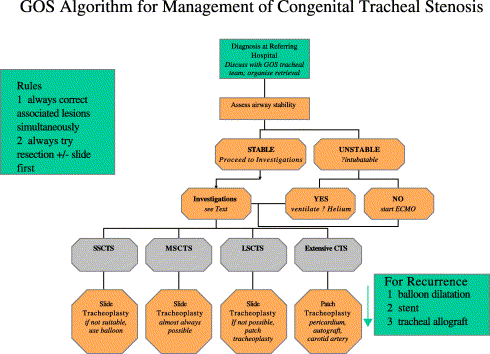
It is both possible and worthwhile to treat long-segment tracheal stenoses in children. Results are improving fast, and new, sophisticated therapies are emerging. Successful strategies for recurrence exist. The treatment algorithm we currently employ is shown as Fig. 2.
Treatment remains both prolonged and expensive. It places a considerable strain on families. However, compared with watching your child try to die and suffer the terrible symptoms of a narrow airway, the vast majority of families are delighted that some treatment is available.
We must do better. Mortality must fall, and morbidity must diminish. This is only likely to happen by concentrating skills and referrals and maintaining a multidisciplinary team approach.