Abstract
The lymphoid tissue of Waldeyer's ring, and particularly the nasopharyngeal tonsil (adenoids), appears to be functionally comparable to nasal-associated lymphoid tissue in rodents. Antigen-stimulated lymphoid follicles give rise to: (a) clonal B-cell expansion; (b) B-cell receptor affinity maturation; (c) positive selection of B cells according to receptor affinity for antigen; (d) differentiation to B memory cells and plasma cells; and (e) variable induction of the joining (J)-chain gene. B-cell differentiation is also important to promote downstream isotype switching of the immunoglobulin (Ig) heavy chain constant genes. For tonsillar B cells, this process gives rise mainly to IgG and IgA plasma cells, partially associated with J-chain expression. Because the J chain is a key peptide in the polymer structure of secretory IgA, tonsils and adenoids may provide B cells for mucosal effector sites. Thus, several observations suggest that these lymphoid organs generate polymeric IgA (pIgA)-expressing B cells that migrate to the upper airway mucosa, lacrimal glands and salivary glands. Accordingly, the nasal route of vaccination induces secretory IgA-dependent regional mucosal immunity and will also enhance systemic immunity. Although the pIgA-producing capacity of tonsillar B cells is considerably decreased in children with recurrent tonsillitis, a conservative attitude towards adenotonsillectomy appears immunologically desirable, particularly in the young age group.
E-mail address: [email protected]
Popular choices
- Casinos Not On Gamstop
- Casinos Not On Gamstop
- UK Betting Sites
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- Non Gamstop Casinos UK
- Slots Not On Gamstop
- Sites Not On Gamstop
- UK Casino Not On Gamstop
- Non Gamstop Casinos
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- Casinos Not On Gamstop
- Top UK Slot Sites
- Casinos Not On Gamstop
- Non Gamstop Casino Sites UK
- Sites Not On Gamstop
- Casino Sites UK Not On Gamstop
- Casinos Not On Gamstop
- Casino Sites Not On Gamstop
- UK Casinos Not On Gamstop
doi:10.1016/S0531-5131(03)00964-6
Click here for the PDF version
Contents
1. Introduction
The nasopharyngeal tonsil (adenoids) and palatine and lingual tonsils constitute the major part of Waldeyer's ring, with the tubal tonsils and latteral pharyngeal bands as less prominent components [1, 2]. These lymphoepithelial elements appear to be functionally comparable to the nasopharynx-associated lymphoid tissue (NALT) in rodents, which is an organized lymphoid structure present on both sides of the nasopharyngeal duct dorsal to the cartilaginous soft palate [3, 4]. All parts of Waldeyer's ring are indeed strategically located to perform regional immune functions because these structures are exposed to both airborne and alimentary antigens. However, although tonsils and adenoids apparently play an important immune-inductive role as components of mucosa-associated lymphoid tissue (MALT), these structures also show similarities with lymph nodes and may in addition participate as effector organs of local systemic-type as well as mucosal-type of adaptive immunity.
Tonsils and adenoids contain four specialized lymphoid compartments participating in the immune functions of these organs [5, 6], namely the reticular crypt epithelium, the extrafollicular area, the mantle zones of lymphoid follicles and the follicular germinal centres (GCs). Primary follicles are present in human tonsils as early as at 16 weeks of gestation [5], which is similar to Peyer's patches of gut-associated lymphoid tissue (GALT) but different from rodent NALT, whose organogenesis begins at birth [4]. Nevertheless, the formation of tonsillar GCs that reflects B-cell activation induced by exogenous antigens does not take place until shortly after birth, and terminal differentiation of effector B cells to extrafollicular plasma cells can first be seen approximately 2 weeks postnatally [5].
The GCs characteristically arise in T cell-dependent B-cell responses and are associated with: (a) clonal expansion of B cells; (b) somatic hypermutation in B-cell immunoglobulin (Ig) variable (Ig V)-region genes; (c) positive selection of B cells that are able to receive antigen-specific signals by high affinity; (d) subsequent differentiation to B memory cells and plasma cells of various isotypes; and (e) induction of the J-chain gene in a variable subset of B cells. This gene encodes a 15-kDa peptide, the J (joining) chain, which is a crucial structural part of polymeric immunoglobulins (pIgs) – that is, dimers and larger polymers of IgA (collectively called pIgA) and pentameric IgM [7]. Without the incorporation of J chain, pIgs cannot bind to the transmembrane epithelial secretory component (SC), which acts as the pIg receptor (pIgR); this interaction is a central step in the formation and selective external transport of secretory IgA (SIgA) and secretory IgM (SIgM) antibodies.
This review deals with the immunological function of tonsils and adenoids and discusses accumulating evidence for a putative role of these structures in secretory immunity of the upper respiratory tract and associated glands. Results are indeed convincing to support the notion that pIgA precursor cells disseminate from Waldeyer's ring to regional mucosal effector sites such as the nasal mucosa and lacrimal and salivary glands [6, 7, 8, 9]. Let me add that the ambitious title of this review was given to me by the congress organizers.
2. Germinal centres as B cell-inductive lymphoid compartments
Primary follicles of secondary lymphoid organs such as the tonsils and adenoids, consist mainly of recirculating B cells with a naïve phenotype positive for surface IgM and IgD (sIgM+sIgD+) – both isotypes exhibiting the same specificity for antigen. These lymphocytes pass into the spaces of the network formed by follicular dendritic cells (FDCs). It is still unclear why both sIgM and sIgD need to be expressed to render B cells antigen-reactive [10]. Likewise, the nature and origin of FDCs are obscure, but the existence of these cells and their accumulation in the primary follicles depend on the presence of B cells. Thus, animals depleted of B cells, or mice with severe combined immunodeficiency (SCID), do not have follicular aggregates of FDCs [11].
In contrast to lymph nodes, tonsils and adenoids lack afferent lymph, but the reticular crypt epithelium contains dendritic cells (DCs) that can transport exogenous antigens to the extrafollicular T-cell areas and to the B-cell follicles (Table 1). Interdigitating DCs (IDCs) function as antigen-presenting cells (APCs) in primary immune responses and occur abundantly in the extrafollicular areas, often closely surrounded by T cells, which are mainly of the CD4+('helper') phenotype [5]. These lymphocytes consist of both naïve (CD45RA+) and memory (CD45R0+) subsets, and some express the interleukin-2 receptor (CD25) as a sign of recent activation [12]. Altogether, tonsils and adenoids appear able to mount both primary and secondary T-cell responses.
| Systemic immunity | Mucosal immunity | |
|---|---|---|
| Inductive sites | ||
| Antigen uptake and transport | Ordinary surface epithelia | Epithelia with membrane (M) cells |
| Dendritic cells (DCs) | ||
| Blood circulation | Mucosa-associated lymphoid tissue (MALT): Peyer's patches, appendix and solitary lymphoid follicles (GALT)a | |
| Peripheral lymph nodes, spleen and bone marrow | Tonsils and adenoids | |
| Local (regional) lymph nodes | ||
| Influx of circulating lymphoid cells: adhesion molecules and chemokines/chemokine receptors | Postcapillary high endothelial venules (HEVs) | |
| PNAd/L-selectin (CD62L) SLC (CCL21), ELC (CCL19)/CCR7 | ||
| GALT: MAdCAM-1/a4b7 | ||
| Effector sites | ||
| Homing of memory and effector T and B cells | Peripheral (lymphoid) tissues and sites of chronic inflammation: a variety of adhesion molecules and chemokines/chemokine receptors | Mucosal lamina propria and exocrine glands: MAdCAM-1/a4b7 (gut), other adhesion molecules (? extraintestinal), TECK (CCL25)/CCR9 (small intestine), MEC (CCL28)/CCR10 (? elsewhere) |
| Antibody production | IgG>monomeric | Polymeric IgA>IgM>>IgG |
| IgA>polymeric IgA>IgM | ||
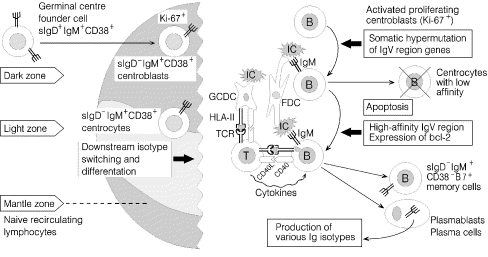
Naïve B cells are initially stimulated in the extrafollicular zones through cognate help from activated CD4+ T cells to which IDCs have presented processed foreign antigen in the context of class II molecules of the major histocompatibility complex (MHC) – in humans also called HLA class II molecules such as HLA-DR. The stimulated B cells can then colonize the primary lymphoid follicles and act as 'founder cells' for GCs; by interaction with the FDCs, which retain native antigen on their surface in the form of immune complexes, the GC founder cells will be induced to proliferate (Fig. 1). A variety of adhesion molecules and other receptor proteins appear to be involved in the interactions that takes place between the B cells and the FDCs [12, 13].
The secondary follicles that result from immune activation can be divided into various more or less well-defined morphological compartments [11]. B-cell stimulation in the GC dark zone gives rise to exponential growth of blasts, which are positive for nuclear proliferation antigen recognized by the monoclonal antibody Ki-67 [12]. The resulting centroblasts hypermutate their Ig V-region genes and give rise to centrocytes (Fig. 1); these cells will die by apoptosis in the GC light zone unless they are selected by their ability to bind with high affinity to antigen present on the surface of FDCs, take up such antigen, and process and present it via HLA class II molecules to CD4+ intrafollicular helper T cells [11].
Terminal complement complex (TCC) occurs on the FDCs [14] as a result of immune complex formation, but this generally causes no apparent harm to the GCs. Perhaps inhibition of C9 polymerization by associated S-protein (vitronectin), protectin (CD59), and decay accelerating factor (DAF or CD55) renders TCC non-lytic [12, 14, 15]. Nevertheless, inflammatory mediators generated by activated complement may cause oedema apparently facilitating formation and dispersion of FDC-derived 'immune complex-coated bodies' or iccosomes and their subsequent uptake by B cells in a receptor-specific manner [12].
Cognate interaction between activated CD4+ helper T cells, which express the costimulatory CD40 ligand (C40L or gp39) and B cells, which express CD40 (Fig. 1), appears to be an important event in the GC reaction [10, 16]. The same is true for the expression of bcl-2 gene products following immune activation of centrocytes to prevent their apoptosis (Fig. 1). Indeed, if the CD40L–CD40 interaction is experimentally blocked, GC are not formed [17]. Importantly, this costimulatory interaction promotes switching of the Ig heavy chain constant (CH) genes of B cells from Cµ (IgM) to downstream isotypes as well as differentiation to plasmablasts and plasma cells secreting high-affinity antibody (Fig. 1). A prerequisite for cognate interaction between B and T cells, in which activated GC B cells present processed antigen to T cells (Fig. 1), is that the former express costimulatory B7 (CD80/CD86) molecules, which can bind to the CD28 receptor on T cells. Classical memory B cells (sIgM+sIgD-) with strong B7 expression are also found extrafollicularly related to the crypt epithelium where they may likewise exert an antigen-presenting function [18].
3. J chain-expressing potential of tonsillar B cells
The tonsillar GC reaction normally generates a variable number of intrafollicular Ig-producing immunocytes (plasmablasts and plasma cells) predominated by cytoplasmic expression of IgG (55–72%) or IgA (13–18%). Both these GC immunocyte classes, and also those producing IgM and IgD, often show concurrent expression of J chain (13–80%), both in normal palatine tonsils and adenoids from children [19, 20]. Thus, the J chain-expressing capacity of IgA immunocytes in these organs is better than that in peripheral lymph nodes, although somewhat less than that in mesenteric lymph nodes, which functionally are extended components of GALT [7, 8, 9].
The cytokine profiles and other microenvironmental factors determining isotype differentiation and coexpression of J chain in B cells remain obscure [8]; it seems that clonal maturation in the course of several proliferative cycles results in reduced expression of J chain, thus promoting monomer production by IgA immunocytes (Fig. 2). Whereas downstream CH gene switching in tonsillar GCs gives rise to a relatively high percentage of extrafollicular IgA immunocytes with J-chain expression (~50%), most extrafollicular IgG immunocytes show little or no such expression (~2%). The fact that the isotype of the former subset is mainly IgA1 (approximately >90%), along with the presence of relatively many IgD immunocytes, supports the notion that tonsillar B-cell differentiation takes place mainly in a classical downstream manner with additional nonclassical switching to IgD [6, 7, 8, 21].
Because the J chain is a key peptide in the formation of pIgA and pentameric IgM that can bind to the pIgR expressed basolaterally on secretory epithelia [7], the tonsillar B-cell differentiation process exhibits features compatible with precursor generation for the SIgA and SIgM system. It may be visualized that only few of the pIgA-expressing plasmablasts that leave the GCs, terminate their differentiation as extrafollicular plasma cells or join the systemic immune system, whereas most of them home to regional secretory effector sites for terminal differentiation there (Table 1, Fig. 2). The adenoids, in addition, possess a local secretory immune system because patches of the crypt epithelium express pIgR/SC, but this is not true for the palatine tonsils [5, 6].
4. Evidence for dissemination of tonsillar B cells to regional secretory effector sites
The finding that nasal and bronchial mucosae, as well as salivary and lacrimal glands, contain an IgA1 and IgD immunocyte distribution similar to that of tonsils and adenoids, supports the notion that these regional secretory effector sites are seeded mainly by B-cell blasts generated in GCs of Waldeyer's ring [6, 7, 8, 9]. A possible minor contribution from bronchus-associated lymphoid tissue remains uncertain, because organized follicles apparently do not exist in the normal human lung [22]. On the other hand, the intestinal lamina propria apparently receives most stimulated B cells from Peyer's patches and other GALT structures such as the appendix and numerous solitary lymphoid follicles [7, 9].
A similar dichotomy of the mucosal immune system has been suggested by B-cell homing studies in the rat [23, 24]. Furthermore, activated human tonsillar B cells were found to migrate to the lung, but not gut mucosa, when transferred to SCID mice [25]. Direct immunization of human palatine tonsils, and particularly nasal vaccination, interestingly gave rise to local B-cell responses in tonsils and adenoids as well as specific circulating B cells that apparently did not enter the small intestinal mucosa [26]. Moreover, in infants dying of the sudden infant death syndrome, the palatine GCs were shown to be overstimulated as revealed by an increased number of IgG and IgA immunocytes [27], and activated B cells apparently disseminated to regional secretory effector sites such as the parotid glands in excessive numbers [28]. It has also been documented in numerous studies that nasal immunization of humans induces specific IgA antibodies in nasopharyngeal secretions, in addition to enhancing systemic immunity [7, 9, 29, 30].
Attempts have been made to track directly the dissemination of B cells from human tonsils and adenoids by means of molecular markers. Because the human herpes virus Epstein–Barr virus (EBV) preferentially establishes persistent infection within memory/effector B cells of Waldeyer's ring, their migration to other compartments may be mapped by DNA analysis for latent EBV infection [31]. On the basis of this approach, B-cell trafficking has been suggested to take place from Waldeyer's ring to peripheral blood and to a lesser extent into systemic lymphoid organs such as mesenteric lymph nodes and the spleen [31]. Using another DNA marker, namely Ig heavy chain Cµ gene deletion [8, 21], we confirmed and extended these results by showing that memory/effector B cells undergoing nonclassical CH switching to IgD immunocyte differentiation, preferentially home from Waldeyer's ring through cervical lymph nodes to the upper airway mucosa and associated glands [32].
Migration of lymphoid cells is strictly regulated by the expression of multiple adhesion molecules and receptors for chemoattractants (chemokine receptors) that interact in a tissue-specific manner with corresponding ligands on endothelial and stromal cells [9, 33]. The extravasation of mainly naïve T and B cells into inductive lymphoid compartments takes place through specialized postcapillary so-called high endothelial venules, and is largely regulated in a similar manner in the systemic and mucosal immune system (Table 1). Conversely, the influx of memory/effector cells into effector tissues through the ordinary local microvasculature is controlled in a much more tissue-specific manner, which is considerably better defined for the small intestinal lamina propria than for other secretory tissues [9, 33]. Thus, the B-cell homing dichotomy between the gut and the upper aerodigestive tract described above clearly has a molecular explanation in terms of adhesion molecules and chemokines, but a detailed delineation of these mechanisms beyond the gut will require further studies (Table 1).
5. Effect of adenotonsillectomy on regional immunity
The observations discussed above provide quite convincing evidence for the theory that Waldeyer's ring functions immunologically as NALT in humans and supply secretory effector sites of the upper aerodigestive region with antigen-stimulated pIgA precursor cells. To further support this notion, it is important to evaluate the effect of adenotonsillectomy on the regional SIgA levels. The pioneer report by Ogra [34] showed that combined tonsillectomy and adenoidectomy in children reduced the level of IgA antibody to poliovirus three- to fourfold in their nasopharyngeal secretions and delayed or abrogated their local immune response to subsequent live oral poliovaccine. Jeschke and Ströder [35] performed tonsillectomy in children and found that their serum Ig and salivary IgA levels decreased for up to 3 years. Furthermore, although D'Amelio et al. [36] observed no salivary IgA reduction (but decreased serum IgA) in previously tonsillectomized adults (16–24 years old), Cantani et al. [37] found in children that salivary IgA as well as serum IgA (and less so IgG and IgM) were significantly reduced 4 months after adenotonsillectomy. More recently, however, studies in tonsillectomized children showed, instead, elevated salivary Ig levels after 3–4 years [38], whereas no effect was found in tonsillectomized young adults after 6 months except for a slight reduction of total IgM and salivary IgG antibodies to Streptococcus mutans and EBV [39].
Altogether, there is a need for more extensive immunological studies focussing collectively on the adenoids and palatine tonsils. Considerable redundancy of inductive lymphoid tissue in Waldeyer's ring might nevertheless mask a potentially unwanted immunological effect of adenotonsillectomy. This possibility is supported by studies that have reported reduced SIgA levels in saliva from children with pharyngitis involving recurrent tonsillitis [40] or adenoid hyperplasia [41]. We have previously documented that repeated tonsillar inflammation (and to a lesser extent adenoid hyperplasia) is associated with decreased J-chain expression by tonsillar B cells, thereby compromising their putative contribution to the regional SIgA system [20]. Especially tonsillitis appears, in an irreversible manner, to speed up the age-related involution of the tonsils as immunological organs [5] in terms of reduced B-cell differentiation to plasma cells (Fig. 3). The underlying immunoregulatory alterations might be related to increased shedding of antigen-transporting M cells in the reticular crypt epithelium, thereby also influencing the regulated balance between expansion of early (J chain-positive) and mature (J chain-negative) effector B-cell clones as discussed elsewhere [5]. Noteworthy, however, is that considerable immunological activity persists even in diseased tonsils and adenoids of children (Fig. 3), so the described functional changes cannot by themselves justify surgical removal of these organs. Therefore, a conservative attitude towards adenotonsillectomy appears immunologically desirable, especially at an early age.