Abstract
Specific language impairment (SLI) is the term used to refer to unexplained difficulties in language acquisition in children. Over the last decade, there has been rapid growth of evidence indicating that genes play an important part in the aetiology of SLI. However, further progress in elucidating the role of genes in causing SLI is limited by our lack of understanding of the phenotype. Studies to date have been hampered by the fact that we do not know whether SLI should be treated as a discrete disorder or a continuous variable, let alone which measures should be used to identify cases, or how many subtypes there are. Recent research suggests that theoretically motivated measures of underlying processes may be better than conventional clinical diagnoses for identifying aetiologically distinct types of language impairment. There has been a tendency for researchers to embrace parsimony and look for a single cause of SLI, or at any event, to identify different subtypes, each with a different single cause. Research is reviewed that suggests that it may not be a fruitful approach to SLI, and that an approach in terms of multiple risk and protective factors, which is widely adopted in medicine, is more realistic.
Reprinted with permission from Philosophical Transactions of the Royal Society, Series B 356:369-380, 2001.
E-mail address: [email protected]
Popular choices
- Casinos Not On Gamstop
- Casinos Not On Gamstop
- UK Betting Sites
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- Non Gamstop Casinos UK
- Slots Not On Gamstop
- Sites Not On Gamstop
- UK Casino Not On Gamstop
- Non Gamstop Casinos
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- UK Online Casinos Not On Gamstop
- Casinos Not On Gamstop
- Top UK Slot Sites
- Casinos Not On Gamstop
- Non Gamstop Casino Sites UK
- Sites Not On Gamstop
- Casino Sites UK Not On Gamstop
- Casinos Not On Gamstop
- Casino Sites Not On Gamstop
- UK Casinos Not On Gamstop
doi:10.1016/S0531-5131(03)00977-4
Click here for the PDF version
Contents
1. Genetic and environmental risks for specific language impairment in children
For most children, language acquisition is fast and easy. Parents find that in the course of a year or so, their babbling infant is transformed into a conversational toddler, with little or no explicit instruction on their part. By 4 years of age most children are able to speak intelligibly in long and complex sentences, drawing upon a vocabulary of hundreds of words. There are, however, children whose language learning does not follow the typical smooth course. Where delayed or deviant language learning has no obvious cause, and where development is proceeding normally in other respects, the term "specific language impairment" (SLI) is used. This is, in part, a diagnosis by exclusion (i.e., the child has language difficulties that are not associated with hearing loss, physical handicap, acquired brain damage, autistic disorder, or more general learning difficulties), and the clinical manifestations can be quite varied. Some children may have obvious difficulties in understanding as well as producing language; others may appear to understand adequately but have problems formulating utterances. There may be limitations of vocabulary, oddities in how language is used to communicate, and/or impairments in producing sequences of speech sounds. A common pattern is for the child to appear immature both in mastery of speech sounds (phonology) and in the correct use of grammatical devices such as inflections, case marking, and auxiliary verbs. Thus, one might see an 8-year-old who produces simple sentences such as "they having a party," or "on Monday I have party, I did." It can be shown that the language difficulties are not part of a more general delay because other aspects of development, such as motor milestones and skills of daily living, are age-appropriate, and performance on nonverbal tests of reasoning and abstraction is normal.
Because language is learned by listening to and interacting with others, it is often assumed that such difficulties must be the consequence of inadequate language input from the child's caregivers. If we rely solely on correlational evidence, we do find environmental factors that differentiate children with SLI from normally developing children. Those with SLI often come from families of lower socioeconomic status [18]. They tend to be younger children from large families [2], and their fathers on average have completed fewer years of formal education [42]. However, these generalisations mask substantial variation within the SLI population, and many children come from affluent homes with well-educated parents. Furthermore, studies that have attempted directly to compare the quality or quantity of maternal speech to children have not reliably found any evidence of communicative inadequacy in parents of those with SLI. Insofar as they do differ from other parents, it seems that they may be modifying their communicative style in response to a language-impaired child [9].
Research over the last decade points to a different kind of explanation for SLI in terms of genetic risk factors for language impairment. I shall first briefly review the evidence, and then go on to consider how behavioural genetic studies can take forward our theoretical understanding of both the nature and the aetiology of SLI.
2. Evidence for genetic influence on SLI
Five types of methodology have been used in this field: family aggregation studies, twin studies, adoption studies, pedigree analysis, and molecular genetics.
2.1. Family aggregation studies
Stromswold [39] reviewed seven family aggregation studies that assessed language impairment in relatives of children with SLI and relatives of a matched control sample. The rates of affected relatives in the control group varied substantially from study to study; this is likely to be a consequence of different criteria used to identify affected cases, and whether only first-degree relatives were included in the computation. Some studies included as affected those reporting reading difficulties, stuttering, or spelling problems, whereas others adopted more stringent criteria. Despite these methodological differences, all studies found a substantial increase in frequency of affected relatives for those with SLI, as compared to a control group. On average, positive family history was found in 46% of those with SLI compared with 18% for controls.
Familial aggregation alone does not provide convincing evidence for genetic influence. It could indicate cultural transmission, i.e., social learning of impaired language patterns by children from their relatives, or the effect of shared environmental influences that are common to family members. Furthermore, parents of a language-impaired child may be particularly alert to language problems in other relatives because they are seeking an explanation for their own child's difficulties. The positive findings from aggregation studies of SLI are not conclusive proof of genetic influence but they provide a strong incentive to do further research on this disorder using more powerful genetic methods.
2.2. Twin studies
Twin studies capitalise on the fact that there are two types of twins who differ in their genetic relatedness. This makes it possible to see how far similarities between children growing up together are a function of their genetic similarity. Many genes do not vary from one person to another, and indeed the majority of human genes are shared with other species. If we wish to explain human variation, rather than species universals, we need to focus on the small proportion of genes that are polymorphic, i.e., different versions of the gene (alleles) are found in different people. Monozygotic (MZ) twins are genetically identical, whereas dizygotic (DZ) twins have on average 50% of polymorphic genes in common. Therefore, if MZ twins are more similar phenotypically than DZ twins, this points to a genetic influence on the phenotype.
Although a great deal of interest has focused on the study of twins reared apart, most twin studies do not involve such unusual cases. Rather, the assumption is made that twin similarity depends both on genetic similarity and on shared environmental influences. Thus, both MZ and DZ twins are expected to resemble each other in language development because they share influences such as noise in the home, type, and quantity of language from caregivers, and exposure to books and TV, as well as sharing the prenatal environment. The critical issue for genetics is not whether twins resemble one another but rather whether MZ twins are more similar to one another than DZ twins. For a dichotomous disorder, where cases can be categorised as affected or unaffected, the statistic of interest involves a comparison of the concordance rate (i.e., proportion of cases where both twins are affected) for MZ and DZ twins. If concordance is significantly higher for MZ twins, then this is evidence that genes play a part in causing disorder.
The logic of the twin method depends on the assumption that both MZ and DZ twins experience equivalent levels of environmental similarity. From time to time twin studies have been criticised on the grounds that this "equal environments" assumption may not be valid. One line of argument is that parents might treat their MZ twins more similarly than DZ twins. It is also possible that shared placental circulation in some MZ twins makes their prenatal environment more similar than that of DZ twins. Insofar as environmental inequalities affect the phenotype of interest, this would mean that twin studies would overestimate the size of genetic effects. The role of such influences can never be ruled out in a twin study, but this does not make such studies worthless, as is sometimes implied. Twin studies need to be interpreted taking into account two factors. First, how plausible is the postulated environmental factor as a causal influence for the phenotype in question? In some studies it may be possible to obtain direct indices of relevant environmental factors within the twin study itself, e.g., by comparing MZ twins who do or do not share placental circulation, or by assessing parental speech to their twin children in relation to zygosity. In other cases, causal links between the environmental factor of interest and the phenotype may be explored in separate studies on single-born children. To date, there has been no success in identifying any environmental factors that are associated with language competence and that are more similar for MZ than for DZ twins, so the equal environments assumption does seem reasonable in the context of SLI. The second point to note is that twin studies need to be complemented by other research methods that make different methodological assumptions. If twin studies give misleadingly high estimates of heritability because they fail to control for differential environments in MZ and DZ twins, then other methods, such as family aggregation studies, pedigree analysis, and adoption studies should show a different picture. In fact, conclusions from studies of SLI using these different methodologies have shown good convergence.
Table 1 summarises data from three twin studies focusing on SLI. All restricted attention to same-sex twins to avoid any confound between zygosity and sex-related effects on SLI. Results from all three studies are in good agreement in finding significantly higher concordance for MZ than DZ twins. This research, taken together with the other studies reviewed in this section, has meant that in less than a decade there has been a radical shift in views about the aetiology of SLI, with widespread acceptance that genes act as a major risk factor for this developmental disorder.
| Zygosity | MZ | DZ |
|---|---|---|
| Lewis and Thompson, 1992 | 0.86 | 0.48 |
| Bishop et al., 1995 | 0.70 | 0.46 |
| Tomblin and Buckwalter, 1998 | 0.96 | 0.69 |
2.3. Adoption studies
In theory, adoption provides a natural experiment for studying the relative importance of genetic and environmental influences on development, by comparing a child's characteristics with those of both the biological and the adoptive parents. In practice, such studies are difficult to carry out because biological parents may be difficult to trace and unwilling to participate in research. Furthermore, interpretation is not always straightforward because similarities between a child and the biological parent could be due to very early, possibly prenatal, environmental influences, rather than to genetic similarities. Nevertheless, adoption provides a valuable opportunity to assess the possible role of the child's home language environment in causing speech and language difficulties. Felsenfeld and Plomin [14] selected two groups of adopted children on the basis of self-reported speech problems in parents. Sixteen children had a biological parent who had a speech disorder (biological risk group), and 19 children had an adoptive parent with a speech disorder (environmental risk group). In addition, 31 nonadopted children with an affected parent (dual risk group) and 90 nonadopted children with both parents unaffected (no risk group) were studied. Rates of speech impairment in the children were 31% for the biological risk group, 11% for the environment risk group, 23% for the dual risk group, and 9% for the no risk group. Overall, having an affected biological parent significantly increased the risk of a child having speech problems, whereas living with an affected parent did not. Such results are compatible with either genetic influence on speech disorder, or early prenatal influences.
In interpreting these results, it should be noted that the phenotype that was the focus of the Felsenfeld and Plomin study was not defined in terms of diagnostic criteria for SLI. Affected status in parents was determined by a series of questions that focused on speech (articulation problems and fluency) rather than language difficulties. Similarly, in the children, affected status was coded partly in terms of the clarity and fluency of the child's speech rather than on language complexity or maturity. Nevertheless, 7 of 11 affected children had received therapy for language problems extending beyond speech production, suggesting that there was some overlap with SLI.
2.4. Pedigree studies
Twin and adoption studies can provide evidence that genes are implicated in a disorder but they tell us nothing about the mode of transmission. In principle, we can obtain information by considering the segregation of a disorder within an extended family. However, pedigree analysis requires clear-cut classification of individuals as affected or unaffected, and in practice, it is fraught with difficulties when dealing with a condition that varies in degree, lacks clear diagnostic criteria, and whose manifestation may change with age. Fig. 1 shows the distribution of language impairment in the three-generation KE family whose linguistic, behavioural, neurological, and genetic characteristics have been described by several research groups [13, 23, 25, 46, 47]. The pedigree shows that the probability of SLI in the offspring of an affected individual is approximately 0.5, exactly as would be predicted if a single dominant gene were implicated in the aetiology. Severe SLI is around three times more common in males than females [24, 30, 37], which leads one to speculate whether some kind of sex-linked inheritance might be implicated. However, the pattern of inheritance in this family is not compatible with that line of explanation: the disorder is equally likely to be transmitted via males and females.
The question arises as to whether one can generalise from the KE family to conclude that a single autosomal dominant gene causes all cases of SLI. Stromswold [39] garnered from the literature five additional pedigrees, none of which is as clear-cut as the KE family, insofar as one finds affected offspring of unaffected parents, or unaffected offspring of two affected parents. Furthermore, Tomblin [43] found that just over half of his sample of 44 children with SLI had no affected first-degree relative. Such departures from classic Mendelian patterns of segregation could reflect misclassification of family members, aetiologic heterogeneity (with some cases due to polygenic or nondominant inheritance, or to environmental factors), or it could indicate that a single dominant gene leads to a risk for SLI that is only manifested under certain environmental conditions (incomplete penetrance).
2.5. Molecular genetics
A pedigree such as that of the KE family (Fig. 1) provides a perfect opportunity for doing genetic linkage analysis, i.e., mapping the genes of affected and unaffected family members to identify chromosomal regions that cosegregate with SLI. Linkage analysis does not identify the specific gene that is implicated in SLI; rather, it relies on the fact that genes close together on a chromosome are likely to be inherited together, to home in on the relevant region of the chromosome. Fisher et al. [16] found linkage of SLI in the KE family to a region on the long arm of chromosome 7 (7q31). More recently, Lai et al. [26] have narrowed the region of interest further. It is intriguing to note that this region of chromosome 7 has also been implicated in the genetics of autism [17].
3. Behavioural genetic research on SLI: current directions
Does the success of studies such as that by Fisher et al. [16] mean that behavioural science has fulfilled its role, and can now pass the problem over to the wet lab? I would argue not: as Michel and Moore [29] have aptly stated: "the discovery that a behavioural pattern can be influenced by genetic manipulations should be viewed more as a beginning of psychological inquiry than as the end" (p. 52). Behavioural studies remain important for two reasons. First, as noted above, SLI is a heterogeneous condition, defined largely in terms of exclusionary criteria. Any search for genes affecting SLI depends crucially on having an appropriate definition of the heritable phenotype. Studies to date have been hampered by the fact that we do not know whether SLI should be treated as a discrete disorder or a continuous variable, let alone which measures should be used to identify cases, or how many subtypes there are. Second, in our excitement at finding genetic influences on SLI, we should not forget the environment. As Plomin et al. [34] pointed out, genetically informative research designs can provide some of the best evidence for nongenetic influences on disorder. In the remainder of this paper, I aim to show how behavioural studies continue to play a critical role in research on causes of language disorder, focusing on three central questions: (i) What measures are most appropriate for identifying a heritable phenotype of SLI? (ii) How can we identify environmental factors that are implicated in the aetiology of SLI? (iii) And, should SLI be regarded as a discrete disorder or the extreme end of a continuum?
3.1. Identifying the heritable phenotype
Most research on the aetiology of SLI has relied on conventional clinical diagnostic criteria to identify affected individuals. However, as noted above, this is often unsatisfactory, leading to uncertainty as to whom to treat as affected or unaffected. Data from the twin study by Bishop et al. [7] illustrate this point. The definition of SLI adopted in that study was based on diagnostic criteria proposed by the American Psychiatric Association [1]. Most children classed as "affected" scored below the 10th percentile on at least one of four standardised language tests, and had a substantial mismatch between poor language and average or good nonverbal ability. A few additional children had pure phonological difficulties, making many errors on an articulation test despite normal intelligence and physical status. These two subgroups are shown together in Fig. 2 as cases of "specific speech/language disorder." It was not uncommon to find cases of MZ twin pairs where one twin was classified as affected, but the other was not, despite having evidence of speech or language difficulties. In one case, the cotwin of an affected child had an IQ below 70 (shown separately in Fig. 2 as "low IQ"). In most cases, however, the cotwin had a nonverbal IQ within broadly normal limits but the mismatch with language ability was not substantial. For instance, a child who had a nonverbal IQ of 85 and language test score of 75 (both being measured on a scale with mean of 100 and S.D. of 15) would not have met criteria for SLI, though his language is clearly below normal limits. Such cases are indicated in Fig. 2 as cases of "low language." Yet, other cotwins had a clear history of speech and language difficulties but performance on the language tests was normal when the child was assessed. These are indicated as 'resolved' cases. Fig. 2 shows how concordance between MZ twin pairs approaches 100%, and that for DZ twins approaches 50%, if "low language" and "resolved" cases are included as "affected." This is just the pattern we should expect to see if genes were overwhelmingly important in the aetiology. Should we therefore relax our criteria for SLI and treat as affected any child who has indications of language difficulties at some point in development? That would seem a poor strategy for identifying an aetiologically homogeneous category, given the wide phenotypic heterogeneity in SLI.
A radically different approach is to move away from clinical diagnostic categories and to assess SLI in terms of the putative underlying impairment. We are a long way from identifying the biological processes underlying SLI, but we have several competing accounts of underlying cognitive mechanisms. Bishop et al. [4] used measures derived from two theoretical accounts of SLI in a twin study with the aim of arriving at a better characterisation of the heritable phenotype.
The first theory, originally proposed by Tallal and Piercy [41], and subsequently developed over many years of research (see Ref. [40]), maintains that SLI is the consequence of limited temporal resolution in the nervous system. In essence, the theory proposes that individuals vary in the rate at which they can process incoming information, and where the rate is slow, they will fail to discriminate between stimuli that are brief or rapid. The deficit is seen as affecting all modalities but has particularly severe consequences for speech perception, which requires the child to distinguish sounds that are of short duration and occur in rapid succession. In support of this theory, Tallal et al. have amassed a wealth of data showing that children with SLI have difficulties in discriminating between auditory stimuli that are brief or rapid, regardless of whether the stimuli are speech sounds or meaningless tones.
The second theoretical account, proposed by Gathercole and Baddeley [19], was developed from studies showing that language learning depends on phonological short-term memory, a specialised system for retaining sequences of speech sounds for brief periods of time. Gathercole and Baddeley assessed phonological short-term memory using a task of nonword repetition in which the child hears meaningless sequences, such as "blonterstaping" or "perplisteronk," and simply has to repeat them back. Ability to do this task accurately predicts vocabulary growth in normally developing children, and is severely impaired in children with SLI (see Ref. [20] for a review). However, this evidence is not inconsistent with Tallal's temporal processing account. It could be that nonword repetition is difficult precisely because it requires the child to discriminate rapid sequences of speech sounds. The study by Bishop et al. [4] aimed to see whether problems in auditory processing and nonword repetition were part of the same underlying heritable disorder.
Children participating in the study were recruited from two sources. Sample A was selected on the basis that the twins had taken part in the earlier study by Bishop et al. [7], and one or both of them had met clinical criteria for SLI. Sample B was a general population twin sample. All twins in both samples were from same-sex pairs. These children, who were aged from 7 to 13 years, were given a battery of standardised tests of language and nonverbal ability, as well as experimental measures designed to tap critical abilities relevant to the two different theoretical accounts.
The test of auditory temporal processing was the Auditory Repetition Test (ART), devised by Tallal et al., which assessed the ability to discriminate sequences of tones presenting slow or fast presentation rates. The child first learns to press one button on hearing a low tone and another on hearing a high tone. Once this association is mastered, tone sequences, varying in length and rate, are presented, and the child is asked to press the sequence of buttons to match the tones. The prediction from Tallal's previous work was that children with SLI should do worse than control children overall on this task and be disproportionately poor with rapid sequences.
To measure phonological short-term memory we used the Children's Test of Nonword Repetition (CNRep), developed by Gathercole et al. [21]. In previous research, they showed that children with SLI do not differ from control children in repeating two-syllable nonwords but they do significantly worse as the number of syllables increases to four or five.
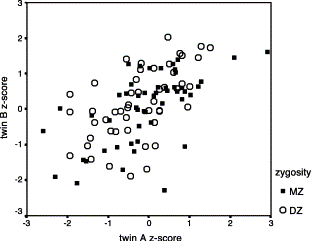
The interest was not just in establishing heritability of different abilities but also in seeing how far the different indices of language impairment clustered together. We had expected that ART and CNRep might turn out to be alternative ways of measuring the same underlying problem but the genetic analyses told quite a different story. Both tests discriminated significantly between language-impaired and control children, though the difference was more striking for CNRep than for ART, and children with SLI were equally poor on fast and slow ART sequences rather than showing the predicted selective deficit on fast sequences. Of particular interest was that the pattern of twin–twin correlations was different for the two tests, as can be seen in Figs. 3 and 4. For CNRep (Fig. 3), the correlation was substantially higher for MZ than for DZ twins, indicating significant heritability, whereas for ART (Fig. 4), twins tended to resemble one another, but this was equally true for MZ and DZ twins, whose twin–twin correlations did not differ significantly. The pattern of results on ART thus suggested that variation in auditory temporal processing was solely due to environmental factors and not affected by genetic influences. We can use the correlations in sample B to obtain a rough estimate of heritability for individual differences in the normal range, by doubling the difference in correlations between MZ and DZ twins.
An alternative method [12] can be used to assess heritability of extreme scores, also known as group heritability, (hg2). Here one starts by defining as probands those children whose test score falls below a cut-off: in this study, children who scored more than 1 S.D. below the mean were selected, with separate analyses being conducted for ART and CNRep. Multiple regression is used, with the probands' scores as predictor variable, and the cotwins' scores as dependent variable. An estimate of hg2 is obtained by considering how much prediction is improved by including an index of genetic relationship (1.0 for MZ twins and 0.5 for DZ twins) as an independent variable. The pattern of results was similar to that seen for the individual differences analysis. For the measures of phonological short-term memory, CNRep, a strong and significant estimate of group heritability (hg2=1.17, S.E. 0.319) was obtained. (The DeFries–Fulker method can lead to heritability estimates outside the range of 0–1; it is likely that these reflect measurement error, though values greater than 1 could also indicate nonadditive genetic effects.) For the auditory temporal processing measure, ART, there was no hint of any genetic influence (hg2=0.109, S.E. 0.324). This lack of genetic effect could not be explained away as the result of using an unreliable measure because there was a strong relationship between the scores of twins and their cotwins. Rather, it appeared that environmental factors shared by two twins were largely responsible for determining whether or not twins were impaired on auditory temporal processing.
The genetic analysis revealed an intriguing dissociation between genetic and environmental influences on different cognitive deficits associated with SLI. How, then, do these causal influences relate to each other? One possibility is that there are distinct subtypes of SLI with genetic and environmental aetiologies. Our data suggested, however, a more complex picture. One can subdivide children according to whether they scored more than 1 S.D. below the mean on (a) the measure of auditory temporal processing, ART, regarded as an index of environmental risk factors; and (b) the measure of phonological short-term memory, CNRep, which is taken as an index of genetic risk factors. Fig. 5 shows the mean language test scores of children subgrouped this way. This figure shows that the difference between those with ART+ and ART- is minimal, suggesting that the environmental risk factor exerts a small influence on language level (significant only for a measure of grammatical understanding). In contrast, the difference between those with CNRep+ and CNRep- is significant for all language measures, suggesting that the genetic risk factor has a larger effect. These influences appear to be additive, so that the children who do worst are those who have both risk factors present (ART- CNRep-). Even so, the relationship between a clinical diagnosis of SLI and poor performance on these tests is far from perfect: the percentages with a diagnosis of SLI were 12% for those with no deficit on ART or CNRep, 18% for those with a deficit on ART only, 36% for those with a deficit on CNRep only, and 54% for those with a deficit on both measures.
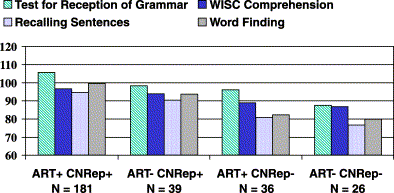
This study emphasises that genetically informative designs are not just useful for finding out about the causes of disorder: they may also help us clarify causal relationships between different underlying deficits, and help us arrive at a more coherent definition of the phenotype. It would appear that, at least for school-aged children, auditory and phonological impairments are not just different indicators of the same core disorder; they are distinct deficits with different origins. Furthermore, if we want to trace the molecular origins of heritable language disorder, then a good starting point in our present state of knowledge would be to focus on phenotype definitions that incorporate phonological short-term memory. This research illustrates the point made by Rutter et al. [38] that "history teaches us that the valid phenotype and traditional diagnostic concepts rarely coincide exactly." As they noted, we have to start with some definition of the phenotype in order to do genetic research, but we should then consider revising our concept of the phenotype in light of the results, going on to validate our revised concept in new studies.
3.2. Environmental influences on language disorder
The data presented so far suggest that environmental risk factors might play a contributory role in the aetiology of SLI. This raises the question of what those risk factors might be. Environmental influences can be explored by extending the analytic methods used for traditional twin analysis. The goal of such methods is to partition observed phenotypic variance into genetic and environmental components. For many years, the 'environmental' component was regarded as an uninteresting residue, and the focus was simply on estimating heritability (the proportion of phenotypic variation attributable to genetic influence). However, the methods of path analysis allow one to formulate more complex models that incorporate measures of specific environmental influences on behaviour.
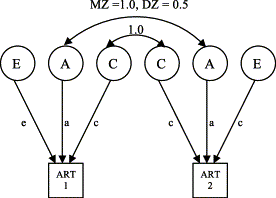
Path analysis involves the use of a path diagram, i.e., a graphic representation of causal and correlational relations between variables. The same information can be represented by a set of simultaneous equations. For illustration, let us take data from 32 DZ twin pairs and 43 MZ twin pairs from Bishop et al.'s [4] sample B on the auditory temporal processing measure (ART). (This is not the full sample. We include here only those cases that have complete data on variables that we shall later incorporate into the analysis.) Our goal is to account for the variance of our observed measures, and the covariance between twin pairs, in terms of underlying latent constructs of genetic and environmental similarity. The standard model for partitioning phenotypic variance into genetic and environmental components is depicted in the path diagram shown in Fig. 6. Observed variables are shown in boxes. These are the ART scores for twin 1 and twin 2 in a pair. Latent variables, i.e., unobserved constructs used to account for the data, are shown in circles. C refers to common, or shared, environment, and represents all those environmental influences that work to make members of a twin pair similar. By definition, these are identical for twin 1 and twin 2 in a pair. This is shown with the curved arrow linking C for twin 1 and twin 2, which indicates that there is a correlation of 1.0 between these variables. The lowercase c denotes one of the values we wish to estimate, namely the strength of the influence of common environment on the language measure. This is identical for both members of a twin pair. The second latent variable is A, which stands for additive genetic variance. (It is possible to model nonadditive genetic influences but these will not be considered here.) The path from A to each twin's score (ART1 and ART2) is denoted by a, which reflects the strength of genetic influence. Because MZ twins are genetically identical, the curved arrow linking A for twin 1 and twin 2 has a value of 1.0 for MZ twins, whereas for DZ twins the value is 0.5. Finally, we include in the model those environmental influences (E) that work to make members of a twin pair different from one another. By definition, there is no relationship between the value of E for twin 1 and twin 2.
Our goal is to estimate the values of c, a, and e from this model from the observed variances and covariances of the twin data. We have six observed statistics; for each zygosity group we have the variance of ART scores for twin 1 and twin 2, and the covariance between ART scores for twin 1 and twin 2. Simple tracing rules are applied to a path diagram to derive the set of equations that yield expected variances and covariances for the variables in the diagram (see Ref. [31]). In the case of Fig. 6, this leads to the two equations:
The variance of the observed measure (ART) is predicted to be the same for twin 1 and twin 2, and for both zygosity groups. In each case:
where variables are standardised, VarP is equal to 1, and so c2, a2, and e2 provide estimates of the proportion of phenotypic variance accounted for by shared environment, genes, and unique environment respectively; thus, a2 is an estimate of heritability (ignoring nonadditive genetic effects). Contemporary model-fitting approaches use iterative routines to find the estimates of the parameters, a, c, and e, that best fit the observed data.
One might wonder why go to all this trouble to obtain a heritability estimate, when much simpler methods, based on comparisons between correlation coefficients, can do the same job. One important reason is that the model-fitting approach allows us to assess goodness of fit of the model using the c2 statistic. In contrast to familiar uses of chi-square, in this instance a high c2 (and low p-value) is undesirable, insofar as it indicates a poor fit between the model and the data. For ART data from Bishop et al. [4], a good fit is obtained (c2 (3)=2.13, p=0.546), but the estimate of the genetic path, a, is 0.
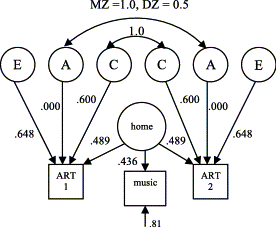
The beauty of the model-fitting approach is that we can extend the model to incorporate observed environmental influences. Fig. 7 shows an extended path diagram that includes a latent variable termed "home," which is used to assess the impact of measured environmental variables. This model was applied to data from Bishop et al. [4] to test the role of different aspects of the home environment. Fig. 7 shows the results of one such test, where "home" is indexed by a simple measure of amount of exposure to live music in the home, based on parental responses to questions about whether anyone in the home played a musical instrument, and the frequency with which live music occurred at home. Is exposure to live music an important environmental factor affecting children's performance on ART? If so, we would expect to find a significant path from "home" to twins' test scores, and a corresponding decrease in the size of the path from C. As can be seen in Fig. 7, when this index of "home" is included in the analysis, the estimate of c drops from 0.774 to 0.600. We can square a path index to obtain an estimate of the amount of variance in a measure due to that path; thus, we can see that the amount of variance explained by C has fallen from 60% to 36%. The difference is accounted for by the new path from "home," which accounts for 24% of the variance in ART scores. In effect, what we are doing in this analysis is seeing how far a measured environmental factor can account for some of the variance that we have so far attributed to the unmeasured, latent variable C. This extended model gives us more explanatory power with no loss of goodness of fit (c2 (6)=4.933, p=0.552). These data are preliminary, the sample size is small for path analysis, and the measure of musical exposure was very crude, but the analysis seems a promising approach for identifying environmental factors that have hitherto been subsumed in the latent variable C. It may prove to be as useful in identifying environmental factors that are not implicated in causing impairments, as in suggesting those that are. Factors that might be expected to influence children's auditory abilities, but which did not give significant paths from "home" when used to index this construct, included social variables such as level of parental education, family size, and single-parent status. Thus, this analysis suggests a rather specific link between exposure to music at home and auditory processing abilities in children, which is not simply a reflection of socioeconomic factors.
I have focused so far on environmental influences that are shared by both twins, and so serve to increase similarity between members of the twin pair. However, as is made explicit in the path analysis, we need also to consider environmental influences specific to the individual, which lead to differences between twins. The E term subsumes both systematic, long-term environmental influences, and also trivial and transient effects that would normally be regarded as "error of measurement." One simple approach to identifying nongenetic factors that are specific to the individual is to look for environmental factors that are related to phenotype in discordant MZ twin pairs. To illustrate this approach, data from the twin study by Bishop et al. [7] were reanalysed. Table 2 shows some comparisons between discordant MZ twin pairs (see Fig. 2) where one twin was affected and the other was either unaffected (N=2) or fell in the "resolved" group (N=15). Medical factors that have been postulated to be related to developmental language difficulties include perinatal risk, otitis media, and allergies (see Refs. [6, 22]), but there was no indication that these were associated with language status in these discordant twin pairs. Nor did twins differ in height, which may be regarded as providing some evidence of nutritional status. At this point, one might start to wonder whether these "discordant" pairs are really so very different, or whether their different categorisation is just a consequence of the fact that categories were defined in terms of language and IQ tests that are inherently unreliable. However, although age at starting speech therapy did not differentiate affected children from their resolved or unaffected cotwins, the affected children continued to receive speech and language therapy for a significantly longer period. This suggests that the difference in severity and persistence of language difficulties was real and not just an artefact of unreliable language measures. This analysis of discordant MZ twins sheds no light on the reasons for the different outcomes in two children who have the same genes and are growing up together, but it does suggest that neither perinatal hazard nor middle ear disease is crucially important.
| Variable | Affected mean (S.D.) | Unaffected/resolved significance mean (S.D.) | |
|---|---|---|---|
| Birth weight (g) | 2613 (462) | 2638 (498) | ns |
| Perinatal risk scorea | 1.24 (1.15) | 1.29 (1.36) | ns |
| Otitis media scorea | 1.24 (1.64) | 1.24 (1.52) | ns |
| History of allergies/eczemab | 0.06 (0.243) | 0.12 (0.332) | ns |
| Height (cm) | 128.3 (13.41) | 129.5 (13.28) | ns |
| Age at starting speech therapy (months) | 44.6 (14.56) | 43.6 (13.72) | ns |
| Duration of speech therapy (months) | 34.2 (26.19) | 23.5 (22.41) | p<0.05 |
3.3. SLI: category or continuum?
One question that has been hotly debated over the years is whether SLI is a qualitatively distinct disorder, or whether it corresponds to the tail of a normal distribution of language ability. One may draw an analogy with height. Some children with short stature suffer from genetic or endocrinological abnormalities that are qualitatively different from normal variation. However, often there is no medical explanation for short stature. Some short children are part of a normal variation, reflecting the fact that any trait that is determined by multiple additive factors will be normally distributed, and any normal distribution must have a tail. Similar issues arise for SLI. Contemporary diagnostic frameworks treat it as a categorical disorder but it is identified on the basis of quantitative test scores, and it is widely recognised that the cut-offs that are used to distinguish normality from disorder are both arbitrary and unreliable (e.g. Ref. [8]). Leonard [27] has argued that there is nothing qualitatively distinct about SLI, and that it should be regarded as the tail end of a normal distribution of language development, affected by the same factors that determine normal variation. In this view, it is futile to look for single major genes in the aetiology. Alternative approaches designed to identify quantitative trait loci would be much more appropriate [34].
It would, however, be premature, to rule out single gene effects. One line of evidence that fits well with the view of SLI as a distinct genetic disorder comes from the pedigree studies reviewed above. In the KE family, both the segregation of the disorder in the family (Fig. 1) and the molecular genetic analysis are consistent with the idea that SLI is caused by a single dominant autosomal gene. The problem, however, is in knowing how far one can generalise from this family, where affected members have a severe disorder affecting both speech and language to an unusual degree. Van der Lely and Stollwerck [45] have presented other family data consistent with autosomal dominant inheritance in children with disproportionate grammatical problems, but in general, it is difficult to distinguish different modes of inheritance on the basis of family data [28]. Furthermore, children with the distinctive pattern of grammatical difficulties described by Van der Lely and Stollwerck appear to constitute a tiny minority of the SLI population [5].
If SLI is a distinct disorder, caused by specific defective genes, we would expect to see evidence of stronger heritability at the extreme of language impairment than in the normal range. This was not apparent in the study of CNRep by Bishop et al. [4], but the sample size was too small for a powerful test. A much larger sample was available in a study by Dale et al. [10] who used the DeFries–Fulker method to estimate genetic influences on language impairment, and compared this with estimates of genetic influences on language variation in the normal range in 2-year-old twins. In this study, the investigators approached parents of all twin pairs born in England and Wales over a 1-year period (N=7756) and asked them to report on their children's vocabulary level at 2 years of age, using a well-validated checklist [15]. A total of 3039 families provided data after excluding those who had medical or perinatal problems in one or both twins. Path analysis was used to estimate h2, the proportion of variance that can be accounted for by genetic variation in the whole sample. The estimate of h2 for vocabulary was 0.25, whereas the influence of shared environment was estimated at 0.69. The DeFries–Fulker approach was then used to estimate heritability of extreme scores. Children who used eight or fewer words at the age of 2 years were identified as probands, corresponding to the bottom 5% of the population. This method gave an estimate of hg2 of 0.73, significantly higher than the estimate of common heritability, h2, obtained from the whole sample. This study, then, provided the first clear evidence that genetic influences play a more substantial role in accounting for language level at the extreme low end of the distribution than across the rest of the range. We need to be cautious in extrapolating from these data on vocabulary level in 2-year-olds to clinically significant SLI. We know that most children with SLI are very slow to start to talk, and would be likely to be picked up as having poor vocabulary at 2 years. However, many late-talking 2-year-olds turn out to be "late bloomers" who do catch up with their peers [32]. Furthermore, these statistical analyses cannot tell us whether the strong genetic influences on language delay reflect the operation of a small number of genes of major effect, or many genes of small effect. The data are, however, compatible with the notion that severe language difficulties may in some cases be influenced by specific genetic defects that are not seen as part of normal variation.
If we accept that at least some types of SLI may be qualitatively distinct, rather than part of normal variation, then this has implications for how we diagnose this condition. Traditionally, diagnosis has been based on psychometric tests that have been designed to give maximum discrimination between individuals in a population, and to yield scores on an interval scale. However, to identify a qualitatively distinct disorder reliably, we require a different kind of measure that will act as a phenotypic marker. If we extend the analogy with growth disorders, we can see that to identify those cases whose small stature has a distinct medical cause, we need some external indicator other than height itself, such as endocrine or chromosome tests. Likewise, if there are distinct causes of poor language that are not part of normal variation, we should be able to find distinctive characteristics that are qualitatively different from normality. The ideal measure would be one that was bimodally distributed, with no overlap in the range of scores for affected and unaffected individuals. We would not be interested in discriminating between individuals within the unaffected range, so it would not matter if they obtained scores at the ceiling. In addition, we would have no concern for the "ecological validity" of the measure. Usually, when devising a language test, the goal is to measure skills that are important for everyday life, so one can identify children whose language impairment might interfere with academic progress or everyday communication. If our search is for a behavioural marker of heritable SLI, however, the most important consideration is how well a measure identifies affected individuals.
Although it is possible that ultimately we may find biological "markers" for SLI, to date researchers have focussed more on qualitative aspects of language itself that might act as a behavioural marker for a distinct phenotype. The past few decades have seen a wealth of studies that aimed to find some aspect of language development in children with SLI that differs from what is seen in the course of normal development. However, years of research on this question have not delivered a convincing answer. In general, language development in children with SLI resembles that seen in younger, normally developing children. On the other hand, the pattern of language development does appear distinctive in SLI, and some aspects of language are differentially vulnerable. For instance, children with SLI tend to do much worse on certain measures of grammatical ability than on vocabulary tests [44]. Rice [36] reviewed evidence showing that on tests of finite verb marking, there is virtually no overlap in the performance of children with SLI and age-matched controls, and she suggested that a test of this ability could act as a useful marker for a heritable phenotype. Genetic studies using such measures are currently underway.
4. Future directions
Where will research on the causes of SLI be in 20 years time? The optimistic view is that, if we develop better methods for identifying behavioural markers that correspond to discrete subtypes of disorder, we will be able to move ahead rapidly to discover specific genes implicated in the aetiology. However, research on other medical disorders offers a note of caution and suggests that progress may not be so swift. Even when we think we have a single diagnostic entity with clear boundaries, and where genes are playing a major role, underlying genetic causes can be remarkably diverse. It is sobering to reflect that, whereas SLI researchers are currently seeking markers to identify qualitatively distinct subtypes of disorder, for many other medical and behavioural disorders, the trend has been in the opposite direction, in favour of models that postulate a continuously distributed underlying liability to disorder, in place of discrete categories [33].
Furthermore, as Plomin and Rutter [35] have pointed out, few medical disorders are caused by a single genetic defect. We are more likely to find multiple genes operating in a probabilistic manner as risk factors, in conjunction with environmental influences. The findings of Bishop et al. [4] are compatible with such a conceptualisation of SLI. The measure of phonological short-term memory, nonword repetition, was heritable in the normal range, as well as at the lower extreme. Those with low scores on this measure had a raised probability of being categorised as having current or resolved SLI, but there was not a sharp divide between impairment and normality. Furthermore, there was evidence that environmental factors play a part in determining whether a child has clinically significant SLI.
There has been a tendency for researchers to embrace parsimony and look for a single cause of SLI, or at any event, to identify different subtypes, each with a different single cause. Research reviewed here suggests that may not be a fruitful approach to SLI, and that an approach in terms of multiple risk and protective factors, which is widely adopted in medicine, is more realistic. In this view, the relationship between causal factors and disorder is probabilistic rather than deterministic. We would not, for instance, reject a causal role for cigarette smoking in the aetiology of lung cancer just because we find nonsmokers who contract lung cancer, or smokers who remain healthy. The data of Bishop et al. [4] suggest that poor performance on a test of nonword repetition indexes a genetic risk factor that is more common in those with SLI than in those without the disorder, but which may not lead to clinically significant language impairment unless other risk factors are also present. Auditory temporal processing deficits appear environmentally determined and exert a relatively small influence on language level, which is not usually of clinical importance. However, when this environmental risk is present in a child who is already at genetic risk, the cumulative effect is to raise the likelihood that the child will meet clinical criteria for SLI. Another way of looking at these findings is to argue that a single risk factor alone is unlikely to be sufficient to cause a major language problem; language development is usually resilient but will be affected if compromised by a combination of adverse influences. This more complex view of the aetiology of SLI suggests that progress in unravelling causes will be slow, and that it will be necessary to take into account environmental as well as genetic influences on disorder.
5. Implications for intervention
As Dawkins [11] noted, the discovery that a disorder has a genetic basis is often seen as a reason for a gloomy prognosis: "if it is in the genes it is 'written', it is 'determined' and nothing can be done about it" (p. 13). This conclusion, as Dawkins points out, reflects a misunderstanding of genetics that is all too common. A heritability estimate does not reflect a fixed value; it is specific to the population under study, and depends crucially on the amount of environmental variation in that population. Suppose we study genetics of reading impairment. We are likely to find higher heritability estimates if we restrict consideration to children who are all receiving optimal instruction in reading, and come from homes where reading is encouraged, than if we include a broader social mix of children, including some who are frequently truant, have few books at home, and are poorly taught. In the latter case, the proportion of variation in reading ability due to environmental factors is likely to be higher, and heritability estimates will be correspondingly lower (see Ref. [3]). Furthermore, a heritability estimate tells us nothing about the possible impact of environmental experiences that are not normally encountered in the population at large, such as specific interventions for language or literacy problems. It is sometimes thought that a genetic influence on a behavioural disorder makes intervention pointless. This is far from being the case. When we find evidence for limited environmental influence on SLI, this tells us that the kinds of variation in environment experienced by most children are likely to have little effect on their language disorder, and that if we want to make a difference, we need to develop specific interventions that target the underlying problem. Nobody suggests this is easy, but it is not a futile aim.